Company Profile
-

Company profile
-
Sophonix Co., Ltd. was founded in July 2017 and the headquarter is located in Beijing Economical and Technological Development Zone. The company has established dual R&D centers in California and Beijing respectively. The core team possess over 15 years of expertise and experience in the IVD field and are dedicated to providing more convenient, accurate and reliable diagnosis services and products through technological innovations.
The company has a complete product portfolio of independent intellectual property rights, including: automatic chemiluminescence immunoassay analyzers (CLEIA method), pathological diagnosis system (Automatic Immunohistochemical Staining Machine) and molecular diagnosis system (Isothermal Nucleic Acid Amplification Analyzer, LAMP technology) and a broad panel of the compatible tests, which are suitable for POCT rapid detection sites.
-

Product introduction
-
The chemiluminescence immunodiagnosis product line consists of MS-Fast, Aceso 80A, MS-Fast pro 80/160/240 series instruments and the compatible magnetic particle chemiluminescence reagent kits.
The test items cover a wide range of fields including: traumatic brain injury (TBI), SARS-CoV-2 neutralization antibody, IgG/IgM antibody, antigen, cytokine storm, alzheimer disease, tumor markers, cardiovascular & cerebrovascular disease, maternal and child care, inflammation markers, thyroid function, diabetes/, gonadal hormone, hypertension, gastric function, bone metabolism, etc.
The pathological diagnosis product line is composed of ultra 60 and ultra 30 automatic immunohistochemical staining machines, which complete the full automatic operation of pathological immunohistochemistry and provide strong support for ensuring the accuracy of pathological diagnosis. The machines are widely used in pathology departments and independent detection centers of major traditional Chinese medicine hospitals.
Ultra 60 products have successfully entered a large number of top three-A hospitals and cancer hospitals in China, and have received high evaluations from the customers.
The molecular diagnosis product line, based on lamp isothermal nucleic acid amplification technology platform, has developed GM-fast 60 instrument and the rapid detection reagents targeting at influenza A, B virus, SARS-CoV-2 and other infectious diseases. The upgraded versions are under development to adapt to more application scenarios.
With the continuous development and promotion of primary medical care, Sophonix will continue to plough deep into the field of in vitro diagnosis, so as to make the fully automatic chemiluminescence single test products, pathological diagnosis products and molecular diagnosis products more in line with the market development and customer demand upgrading. Sophonix will be always committed to precision medical to make more people enjoy a healthy life.
Corporate Vision
Popularize the high quality diagnosis through consistent innovation
Company History
Drag the mouse horizontally
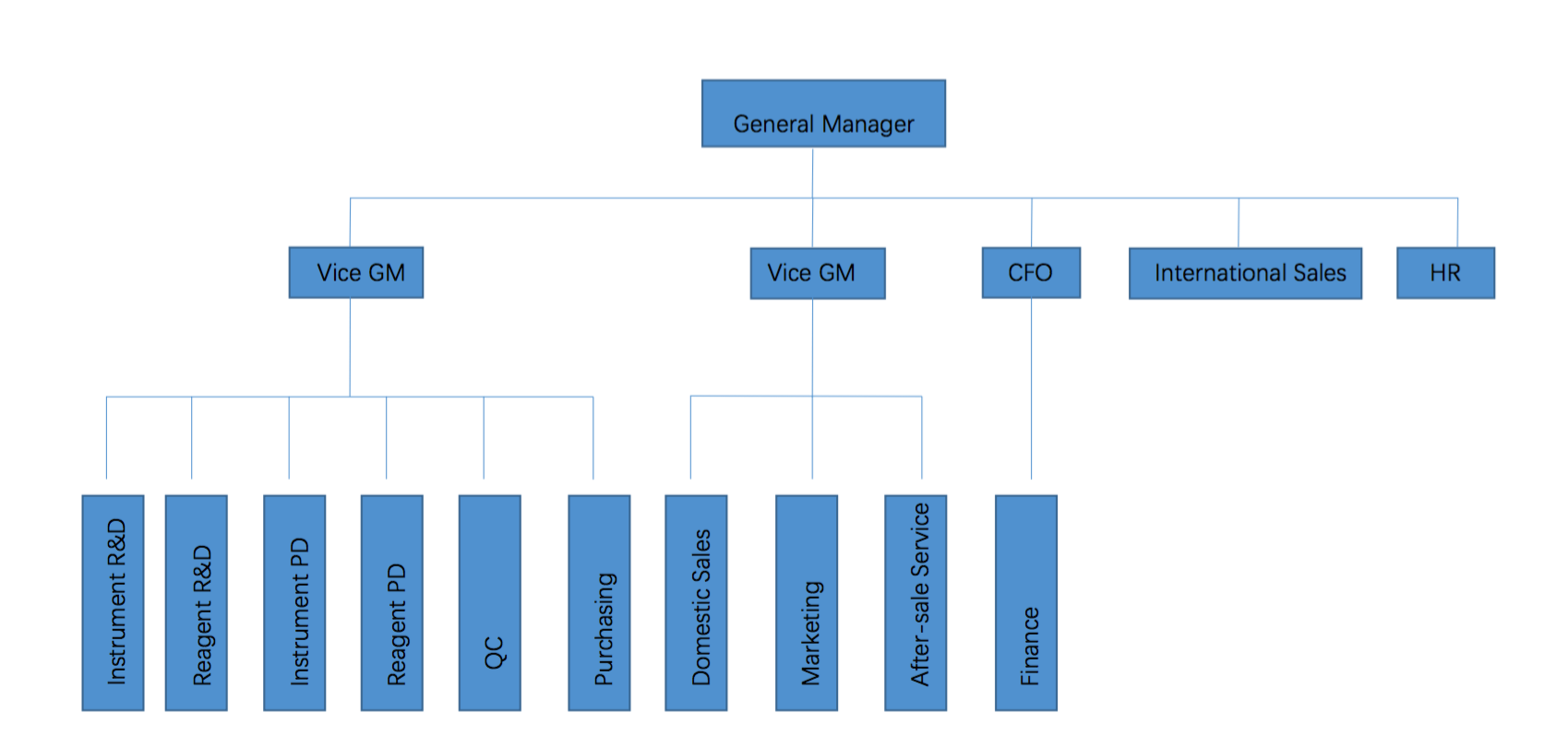
Organization Structure